|
Air & Water Reactions
|
Flammable. Water soluble.
|
|
Reactivity Profile
|
2-Methoxyethanol is incompatible with oxygen and strong oxidizing agents. Contact with bases may result in decomposition. Incompatible with acid chlorides and acid anhydrides. . 2-Methoxyethanol forms explosive peroxides.
|
|
Hazard
|
Toxic by ingestion and inhalation. Moderate fire risk. Toxic by skin absorption. Questionable carcinogen.
|
|
Health Hazard
|
2-Methoxyethanol is a teratogen and a chronic inhalation toxicant. The target organs are blood, kidney,and the central nervous system. In addi tion to inhalation, the other routes of expo sure are absorption through the skin, and ingestion. Animal studies indicated that over-exposure to this compound produced anemia, hematuria, and damage to the testes.In humans, inhalation of EGME vapors cancause headache, drowsiness, weakness, irrita tion of the eyes, ataxia, and tremor. The acuteinhalation toxicity, however, is low and anytoxic effect may be felt at a concentration ofabout 25–30 ppm in air The oral and dermal toxicities of thiscompound in test animals were found to belower than the inhalation toxicity. Ingestioncan produce an anesthetic effect and in alarge dosage can be fatal. An oral intake ofabout 200 mL may cause death to humans. LC50 value (mice): 1480 ppm/7 h, LD50 value (rabbits): 890 mg/kg EGME is a teratogen exhibiting fetotoxi city, affecting the fertility and the litter size,and causing developmental abnormalities inthe urogenital and musculoskeletal systemsin test animals.
|
|
Fire Hazard
|
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
|
|
Flammability and Explosibility
|
Flammable
|
|
Chemical Reactivity
|
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
|
|
Safety Profile
|
Moderately toxic to humans by ingestion. Moderately toxic experimentally by ingestion, inhalation, shin contact, intraperitoneal, and intravenous routes. Human systemic effects by inhalation: change in motor activity, tremors, and convulsions. Experimental teratogenic and reproductive effects. A skin and eye irritant. Mutation data reported. When used under conditions that do not require the application of heat, thts material probably presents little hazard to health. However, in the manufacture of fused collars which require pressing with a hot iron, cases have been reported showing disturbance of the hemopoietic system with or without neurologcal signs and symptoms. The blood picture may resemble that produced by exposure to benzene. Two cases reported had severe aplastic anemia with tremors and marked mental dullness. The persons affected had been exposed to vapors of methyl "Cellosolve," ethanol, methanol, ethyl acetate, and petroleum naphtha. flame. A moderate explosion hazard. Can react with oxidizing materials to form explosive peroxides. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also GLYCOL ETHERS. Flammable liquid when exposed to heat or
|
|
Potential Exposure
|
2-Methoxyethanol is used as a jet fuel additive; solvent for protective coating; and in chemical synthesis. Ethylene glycol ethers are used as solvents for resins used in the electronics industry, lacquers, paints, varnishes, gum, perfume; dyes and inks; and as a constituent of painting pastes, cleaning compounds; liquid soaps; cosmetics, nitrocellulose, and hydraulic fluids.
|
|
Carcinogenicity
|
There are no experimental carcinogenicity or cancer epidemiology data relating to this chemical , but some short-term test data are available and are summarized in the section on genetic and related cellular effects.
|
|
Environmental fate
|
Photolytic. Grosjean (1997) reported an atmospheric rate constant of 1.25 x 10-11 cm3/molecule?sec at 298 K for the reaction of methyl cellosolve and OH radicals. Based on an atmospheric OH concentration of 1.0 x 106 molecule/cm3, the reported half-life of methyl cellosolve is 0.64 d (Grosjean, 1997). Chemical/Physical. At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent concentration of 342 mg/L. The adsorbability of the carbon used was 132 mg/g carbon (Guisti et al., 1974).
|
|
Shipping
|
UN1188 Ethylene glycol monomethyl ether, Hazard Class: 3; Labels: 3-Flammable liquid
|
|
Purification Methods
|
Peroxides can be removed by refluxing with stannous chloride or by filtration under slight pressure through a column of activated alumina. 2-Methoxyethanol can be dried with K2CO3, CaSO4, MgSO4 or silica gel, then distilled from sodium. Aliphatic ketones (and water) can be removed by making the solvent 0.1% in 2,4-dinitrophenylhydrazine and allowing to stand overnight with silica gel before fractionally distilling. [Beilstein 1 IV 2375.]
|
|
Toxicity evaluation
|
High acute doses of methoxyethanol have a sedative and hypnotic effect. Kidney and lung damages, accompanied by hemoglobinuria, follow exposures to high doses. Toxicity is attributed to the active metabolites: methoxyacetaldehyde and methoxyacetate. In vitro studies with radiolabeled methoxyethanol indicate that formation of methoxyacetyl-coenzyme A may lead to the formation of methoxyacetyl derivatives of Krebs cycle intermediates. Methoxyacetate produces the same testicular lesions in rodents as does the parent compound, although the immunosuppression elicited by methoxyethanol exposure may depend on the putative metabolite, methoxyacetaldehyde. In both the testicular lesion and the immune suppression, some data suggest that the pattern of cell death termed ‘apoptosis’ may be stimulated. Methoxyacetate stimulates synthesis of progesterone by luteal cells in culture. This disturbance of luteal function may be related to the prolongation of gestation in rodents. Teratogenicity appears to be related to interference by methoxyethanol, or its metabolites, with one carbon metabolism in the synthesis of nucleotide precursors, and can be relieved by administration of other substrates, such as serine and glycine, which also provide substrates for nucleotide synthesis. It has also been suggested that toxicity is mediated through inhibition of flavoprotein dehydrogenase-catalyzed reactions.
|
|
Incompatibilities
|
Vapors may form explosive mixture with air. Heat or oxidizers may cause the formation of unstable peroxides. Attacks many metals. Strong oxidizers may cause fire and explosions. Strong bases cause decomposition and the formation of toxic gas. Attacks some plastics, rubber and coatings. May accumulate static electrical charges, and may cause ignition of its vapors.
|
|
Waste Disposal
|
Concentrated waste containing no peroxides: discharge liquid at a controlled rate near a pilot flame. Concentrated waste containing peroxides: perforation of a container of the waste from a safe distance followed by open burning.
|
|
Physical properties
|
Colorless liquid with a mild, ether-like odor. Experimentally determined detection and recognition odor threshold concentrations were <300 μg/m3 (<96 ppbv) and 700 μg/m3 (220 ppbv), respectively (Hellman and Small, 1974).
|
|
Definition
|
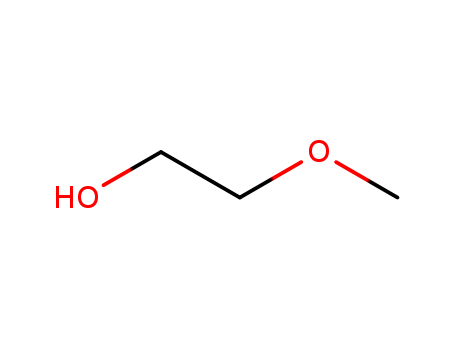
ChEBI: A hydroxyether that is ethanol substituted by a methoxy group at position 2.
|
|
General Description
|
A clear colorless liquid. Flash point of 110°F. Less dense than water. Vapors are heavier than air.
|
中文
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego