|
Outline
|
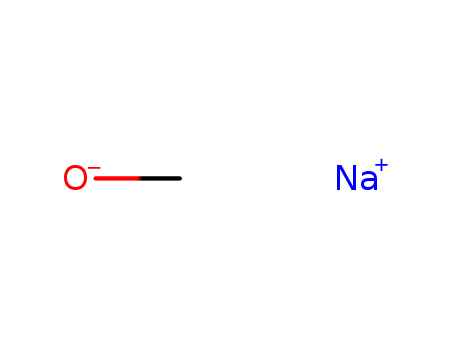
Sodium methanolate is one of alkoxides that are generated by methanol. Its chemical formula is CH3ONa. It is strong base that is commonly used in organic synthesis. Sodium methanolate is often stored in methanol, ethanol or the like alcohol solvent. It can generate suspension when it dissolves in ether. It can decompose into methanol and sodium hydroxide with water: CH3ONa + H2O→CH3OH + NaOH Sodium methanolate product has two forms: solid and liquid. Solid is pure sodium methanolate. The liquid is methanol solution of sodium methanolate and the content of sodium methylate is 27.5% to 31%. Liquid sodium methanolate is colorless or slightly yellow viscous liquid. It is sensitive to oxygen. It is flammable, explosive and easily hygroscopic. It is soluble in methanol and ethanol, and can decompose into methanol and sodium hydroxide with water. It decomposes at 126.6℃ in the air. It is not soluble in benzene and toluene. It is strong irritant and highly corrosive. Sodium methanolate can be used as condensing agent, strong alkaline catalyst and methoxy agent for the preparation of vitamin B1, vitamin A, sulfadiazine and other drugs. Few can be used in production of pesticides. It can also be used as catalyst in processing edible fats and oils (especially processing lard). It is used as analytical reagent. Solid sodium methanolate is colorless amorphous powder. It is sensitive to oxygen, and it is flammable. It is soluble in methanol and ethanol, and can decompose into methanol and sodium hydroxide with water. It decomposes at 126.6℃ in the air. It is mainly used for the production of sulfa drugs, VB6 and VA. Sodium methylate is a kind of catalyst for organic synthesis. It can be used for the production of pesticides and oil processing industries. Solid sodium methanolate and sodium methoxide methanol solution Sodium methylate is a kind of mature chemical products. It has several decades of production history in China. The overall price is high in the south than in the north. Its price is 4500 yuan/T in the southwest, and 4,000 yuan/T in Shandong. Its price changed little with the increase of methanol’s price in 2004. The profit of per ton of product is 500 to 700.
|
|
Soda process for producing sodium methylate
|
Sodium methylate is prepared by the reaction of methanol with sodium hydroxide for soda method. Their reaction is as follows: CH3OH + NaOH→CH3ONa + H2O 1. The preparation of methanol lye Crush the solid sodium hydroxide. Then add it into melting pot base containing methanol (99.8%) in proportion. Start circulating pump, and dissolve the sodium hydroxide below 70℃. When its content is 20% to 23%, cool down to 40℃ and inject into the settling tank. Stand 12h and spare. 2. The preparation of sodium methylate Pass into the steam to the vaporization pot and reaction tower mezzanine. Heat it and control the temperature at 85~100℃. Add anhydrous methanol to vaporization pot at the flow rate of 180L/h. Meanwhile add methanol lye in the top of the reaction tower at the flow rate of 25kg/h. Methanol gas containing 2% of water produced by the reaction from the reaction distill the reaction tower overhead into the purification distillation column to remove water. Then it becomes absolute methanol recycling. Control the temperature of reaction bottoms (i.e. vaporization pot) at 65~70℃, and check the bottoms. When containing 27%~31% Sodium methanolate, 1% or less of the free base, products are obtained. The yield is 86% (as sodium hydroxide). Fixed consumption of the process is as follows: methanol (95%) 973kg/t, sodium hydroxide (99.5%) 209kg/t. The above information is edited by the lookchem of Ge Qian.
|
|
Sodium process for producing sodium methylate
|
Sodium metal and methanol as raw materials, using intermittent production process to produce methanol solution of sodium methylate. The reaction is as follows: 2CH3OH + 2Na→2CH3ONa + H2↑ The production process is mainly divided into four parts: 1. Feeding step. Industrial methanol is pumped into methanol metering tank. 120kg of sodium metal is added into the reaction kettle. 2. Nitrogen substitution step. Close feed valve and vent valve. Purged with nitrogen into the reactor so that the index reached 0.1MPa. Open vent valve to remove the pressure, and repeat three times under reflux. The vent valve remains open to continue introducing nitrogen. 3. Reaction step. The cooling water is fed to the condenser. Methanol is immediately added to the methanol tank (note methanol addition rate). Nitrogen is stopped after 5~10min. Methanol is continuously added. 820kg of methanol is added in 2~3h and then the feed valve is closed. The reaction is continued for 2~3h until the reaction mixture has no bubbles. 4. Reconciliation step. The product is added into the harmonic tank. Sample and analysis. Reconcile to the desired concentration and barrels.
|
|
The comparison soda process with sodium process
|
The content of methanol alkaline get by sodium method is higher, more reliable and stable than alkaline method. And the content of free alkali is low. Sodium methylate prepared by sodium method can take off a lot of alcohol and other impurities from sodium in the reaction. The content of Na2CO3 in sodium method is lower than in alkaline method, which can improve product quality and yield of the users.
|
|
Solution quality reference standard
|
Item Index Appearance colorless to pale yellow viscous liquid Content (total base number) 29.5%~31.0% Sodium methoxide (NaOCH3) 28.9%~31.0% Sodium hydroxide (NaOH) ≤0.5% Sodium carbonate (Na2CO3) ≤0.1% Water (H2O) ≤0.2% Methanol 69.0%~70.7%
|
|
How to separate solid sodium hydroxide and sodium methylate
|
1. Dissolved in water (sodium alcoholate reacts violently with water to generate alcohol and sodium hydroxide) 2. Distillation (separate alcohol) 3. Evaporation (separate sodium hydroxide) 4. Try to dry alcohol to anhydrous state (repeated distillation) 5. Add sodium to generate sodium alcohol 6. Distill alcohol to get solid alcohol sodium. (It should be avoided being exposed to the air when distilling. If it is exposed to the air or it is taken down before drying up and cooling, the state of alcohol sodium that is separated out is not well. The good one is white. if not, it will be tan is. But it doesn’t have any great impact. It is easy to be prepared.)
|
|
Production Process
|
Solid sodium hydroxide and methanol mix at a certain proportion. After stirring the mixture to dissolve, solid impurities are removed by sedimentation and clarification. The clear liquid is pumped into the top of the dehydration reaction column, while the refined methanol vapor is added from the bottom of the tower with continuous dehydration reaction. Then the product can be obtained from tower bottom. Aqueous methanol vapor is purified from the top of the column into the distillation system, while anhydrous methanol can be used repeatedly.
|
|
Identification test
|
It can react with oxygen and carbon dioxide. It decomposes in water. Sodium test of the solution (IT-28) is positive. Take 0.1m1 5% sulfuric acid and 0.2ml potassium permanganate solution (TS-193), and add 1 drop of 1% aqueous solution of the sample. After placing 5min, add 0.2ml 25% sodium sulfite solution and 3ml sulfate. Then add 0.2ml chromium changed acid solution (TS-l66). The solution is amaranth to purple.
|
|
Chemical properties
|
Colorless amorphous fine powder. Dissolved in methanol, ethanol.
|
|
Production method
|
1. Xylene (water content < 0.05) and metallic sodium are added into reaction vessel. Heat the reaction vessel to 130~140℃, keep for 1h and stop heating. After rapid stirring for 1h, use cooling water to 50℃. Then start to dropwise add anhydrous methanol (water content < 0.1%), and appropriately add dry xylene. The dropping rate depends on the flow rate of methanol and the release of hydrogen. After the dropwise addition finishes, heat it under reflux for 4h and cool to room temperature to obtain sodium methanolate slurry. Xylene can be recovered by vacuum distillation and then dried in vacuo for 4h. Sodium methanolate can be obtained by nitrogen cooling. The yield is over 90%. Sodium methanolate can also be prepared by the continuous reaction and dehydration of sodium hydroxide with methanol at 85~100℃. 2. It can be obtained by the reaction of sodium hydroxide and methanol in benzene.
|
|
Category
|
Corrosive materials
|
|
General Description
|
Sodium methylate is a white amorphous powder. It reacts with water to form sodium hydroxide, a corrosive material, and methyl alcohol, a flammable liquid. The heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. It is used to process edible fats and oils, and to make other chemicals.
|
|
Air & Water Reactions
|
Highly flammable. Ignites in moist air [Wischmeyer 1966]. Reacts with water to produce a mixed solution of sodium hydroxide and methyl alcohol.
|
|
Reactivity Profile
|
SODIUM METHYLATE is a strong base. Reacts with light metals forming H2 gas, with fire and explosion hazards. Too rapid addition of sodium methylate to a mixture of chloroform and methanol initiated an uncontrolled exothermic reaction between the chloroform and the methylate that caused a violent explosion [MCA Case History 693 1961]. Sodium methoxide is incompatible with 4-chloronitrobenzene and fluorinated cyclopropenyl methyl ethers, such as perfluoromethoxycyclopropene. The reactions are vigorous and may initiate ignition [Bretherick, 1995, pg. 191].
|
|
Hazard
|
(Solid) Flammable when exposed to heat or flame. (Solution) Flammable, moderate fire risk.
|
|
Health Hazard
|
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
|
|
Fire Hazard
|
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
|
|
Flammability and Explosibility
|
Flammable
|
|
Safety Profile
|
A corrosive and irritating material. It hydrolyzes into methanol and sodlum hydroxide. May ignite spontaneously in moist air. Flammable when exposed to heat or flame. Ignites on contact with water, Violent reaction with (CHCl3 + CH3OH), (methyl azide + dimethylmalonate), FClO3. When heated to decomposition it emits toxic fumes of Na2O.
|
|
Purification Methods
|
It behaves in the same way as sodium ethoxide. It is hygroscopic and is hydrolysed by moist air to NaOH and MeOH. Material that has been kept under N2 should be used. If erratic results are obtained, even with recently purchased NaOMe, it should be freshly prepared thus: Clean Na (37g) cut in 1-3g pieces is added in small portions to stirred MeOH (800mL) in a 2L three-necked flask equipped with a stirrer and a condenser with a drying tube. After all the Na has dissolved, the MeOH is removed by distillation under vacuum, and the residual NaOMe is dried by heating at 150o under vacuum and kept under dry N2 [Burness Org Synth 39 51 1959]. [Beilstein 1 IV 1227.]
|
中文
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego