Enhanced vapor-phase processing in fluorinated Fe4 single-molecule magnets
Rigamonti, Luca,Piccioli, Marco,Malavolti, Luigi,Poggini, Lorenzo,Mannini, Matteo,Totti, Federico,Cortigiani, Brunetto,Magnani, Agnese,Sessoli, Roberta,Cornia, Andrea
, p. 5897 - 5905 (2013)
A new tetrairon(III) single-molecule mag...
A contamination-insensitive probe for imaging specific biomolecules by secondary ion mass spectrometry
Kabatas, Selda,Vreja, Ingrid C.,Saka, Sinem K.,H?schen, Carmen,Kr?hnert, Katharina,Opazo, Felipe,Rizzoli, Silvio O.,Diederichsen, Ulf
, p. 13221 - 13224 (2015)
Imaging techniques should differentiate ...
Scavenging Reaction of Solvated Electron Produced by UV Laser from Iodide Anion in Liquid Beam
Matsumura, Hisashi,Mafune, Fumitaka,Kondow, Tamotsu
, p. 5861 - 5864 (1995)
A sodium iodide (NaI) solution in ethano...
Characterization of new Co(II) complexes and photographic monitoring for their toxic impact on breast cancer cells according to simulation study
Shah, Reem,Habeebullah, Turki M.,Saad, Fawaz,Althagafi, Ismail,Al-dawood, Aisha Y.,Al-Solimy, Amerah M.,Al-Ahmed, Zehba A.,Al-Zahrani, Fatimah,Farghaly, Thoraya A.,El-Metwaly, Nashwa
, (2020)
Five new nitrogen-rich ligands (thioanhy...
Synthesis of new polysubstituted (pyrazoles, pyrimidines and quinolines) five and six-membered heterocycles: Reaction of α,α-dioxoketene dithioacetals with nucleophiles
Ebraheem,Lokanatha Rai,Kudva.n,Bahjat
, p. 3486 - 3492 (2010)
A novel synthesis of polysubstituted pyr...
Synthesis and photophysical properties of europium pentafluorinated β-diketonate complexes
Wan, Yupeng,Lyu, Heng,Du, Hengyi,Wang, Dunjia,Yin, Guodong
, p. 1669 - 1687 (2019)
Two pentafluorinated β-diketone ligands,...
Iron(ii) complexes of 2,6-di(1H-pyrazol-3-yl)-pyridine derivatives with hydrogen bonding and sterically bulky substituents
Roberts, Thomas D.,Little, Marc A.,Kershaw Cook, Laurence J.,Halcrow, Malcolm A.
, p. 7577 - 7588 (2014)
Syntheses of 2,6-di(5-aminopyrazol-3-yl)...
Further studies on the biodegradation of ionic liquids
Ford, Leigh,Harjani, Jitendra R.,Atefi, Farzad,Garcia, M. Teresa,Singer, Robert D.,Scammells, Peter J.
, p. 1783 - 1789 (2010)
A range of ionic liquids (ILs) containin...
The AAAA?DDDD hydrogen bond dimer. Synthesis of a soluble sulfurane as AAAA domain and generation of a DDDD counterpart
Taubitz, Joerg,Luening, Ulrich
, p. 1550 - 1555 (2009)
Sulfurane 5b with solubility enhancing s...
Synthesis and nicotinic activity of epiboxidine: An isoxazole analogue of epibatidine
Badio, Barbara,Garraffo, H. Martin,Plummer, Carlton V.,Padgett, William L.,Daly, John W.
, p. 189 - 194 (1997)
Synthetic (±)-epiboxidine (exo-2-(3-meth...
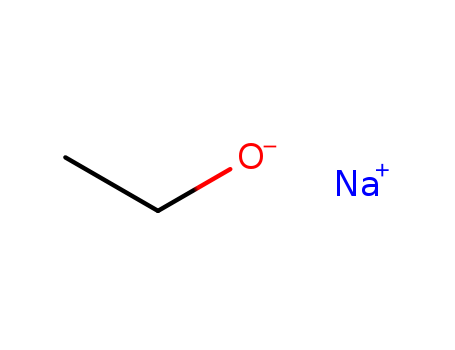
Crystal structure of sodium ethoxide (C2H5ONa), unravelled after 180 years
Beske, Maurice,Tapmeyer, Lukas,Schmidt, Martin U.
, p. 3520 - 3523 (2020)
As early as 1837, Liebig synthesised sol...
Substituent dependence on the spin crossover behaviour of mononuclear Fe(ii) complexes with asymmetric tridentate ligands
Saiki, Ryo,Miyamoto, Haruka,Shiga, Takuya,Oshio, Hiroki,Sagayama, Hajime,Kumai, Reiji,Newton, Graham N.
, p. 3231 - 3236 (2019)
Three mononuclear iron(ii) complexes of ...
Molecular Routes to Group IV Magnesium and Calcium Nanocrystalline Ceramics
Petrus, Rafa?,Dr?g-Jarz?bek, Anna,Utko, Józef,Bykowski, Dominik,Lis, Tadeusz,Sobota, Piotr
, p. 11365 - 11374 (2017)
The effect of alkaline-earth-metal alkox...
Synthesis and characterization of new 5,5′-dimethyl- and 5,5′-diphenylhydantoin-conjugated hemorphin derivatives designed as potential anticonvulsant agents
Georgieva, Stela,Peneva, Petia,Rangelov, Miroslav,Tchekalarova, Jana,Todorov, Petar,Todorova, Nadezhda
, p. 2198 - 2217 (2022/02/16)
Herein, the synthesis and characterizati...
Catalyst system for producing polyethylene copolymers in a high temperature solution polymerization process
-
, (2022/03/27)
Catalyst system for producing ethylene c...
中文
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego