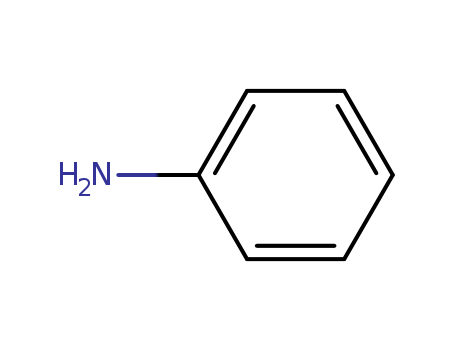|
Reaction
|
A primary aromatic amine, aniline is a weak base and forms salts with mineral acids such as aniline hydrochloride. PKb = 9.30, 0.2mol aqueous solution PH value 8.1. In acidic solution, nitrous acid converts aniline into a diazonium salt that is an intermediate in the preparation of a great number of dyes and other organic compounds of commercial interest. When aniline is heated with organic acids, it gives amides, called anilides, such as acetanilide from aniline and acetic acid. Monomethylaniline and dimethylaniline can be prepared from aniline and methyl alcohol. Catalytic reduction of aniline yields cyclohexylamine.
Various oxidizing agents convert aniline to quinone, azobenzene, nitrosobenzene, p-aminophenol, and the phenazine dye aniline black. Amino groups can undergo acylation, halogenation, alkylation and diazotization, and the presence of amino groups makes it nucleophiles capable of many nucleophilic reactions, and at the same time activates the electrophilic substitution on aromatic rings. |
|
Production
|
Aniline was first obtained in 1826 by the destructive distillation of indigo. It is named because of the specific indigo-yielding plant “Indigofera anil” (Indigofera suffruticosa); In 1857, W.H.Jr. Perkin made aniline from reduction of nitrobenzene with iron filings using hydrochloric acid as catalyst which is still being used. At present, the methods of aniline production include catalytic vapor phase reduction of nitrobenzene with hydrogen, catalytic reaction of chlorobenzene and ammonolysis of phenol (Japan).
Before 1960s, aniline production was based on coal tar benzene, and now petroleum benzene has been used. At the end of 1990s, the world's aniline production capacity was above 2.5 million t. 50% of the aniline is used in the production of dye intermediates. About 25% aniline is used to produce isocyanate and its copolymers. The remaining (25%) is used for pesticides, gasoline antiknock agents, and photographic materials etc. |
|
Hazards
|
The toxicity of Aniline is LD50500mg/kg (dog oral administration), and is a common pollutant in the environment. Aniline has strong toxicity to blood and nerves. It can be absorbed by skin or by respiratory tract to cause toxicity.
The acute (short-term) and chronic (long-term) effects of aniline in humans consist mainly of effects on the lung, such as upper respiratory tract irritation and congestion. Chronic exposure may also result in effects on the blood. Human cancer data are insufficient to conclude that aniline is a cause of bladder tumors while animal studies indicate that aniline causes tumors of the spleen. EPA has classified aniline as a Group B2, probable human carcinogen.
Evidence reported by the National Institute for Occupational Safety and Health (NIOSH) clearly associates the occupational exposure to o-toluidine and aniline with an increased risk of bladder cancer among workers. The risk of bladder cancer is greatest among workers with possible and definite exposures to o-toluidine and aniline, and the risk increases with the duration of exposure. |
|
Physical properties
|
Colorless, oily liquid with a faint ammonia-like odor and burning taste. Gradually becomes yellow
to reddish-brown on exposure to air or light. The lower and upper odor thresholds are 2 and 128
ppm, respectively (quoted, Keith and Walters, 1992). An odor threshold of 1.0 ppmv was reported
by Leonardos et al. (1969). |
|
Production Methods
|
Aniline was obtained in 1826 by Unverdorben from distillation of indigo and was given the name aniline in 1841 by Fritzsche (Windholz et al 1983). The chemical was manufactured in the U. S. by the Bechamp reaction involving reduction of nitrobenzene in the presence of either copper/silica or hydrochloric acid/ferrous chloride catalysts; but in 1966, amination of chlorobenzene with ammonia was introduced (IARC 1982; Northcott 1978). Currently, aniline is produced in the U.S., several European countries and Japan by the catalytic hydrogenation of nitrobenzene in either the vapor phase or solvent system. This chemical is also produced by reacting phenol with ammonia (HSDB 1989). Production in 1982 amounted to 331,000 tons (HSDB 1989). |
|
Definition
|
ChEBI: A primary arylamine in which an amino functional group is substituted for one of the benzene hydrogens. |
|
Synthesis Reference(s)
|
Chemical and Pharmaceutical Bulletin, 29, p. 1159, 1981 DOI: 10.1248/cpb.29.1159The Journal of Organic Chemistry, 58, p. 5620, 1993 DOI: 10.1021/jo00073a018 |
|
General Description
|
A yellowish to brownish oily liquid with a musty fishy odor. Melting point -6°C; boiling point 184°C; flash point 158°F. Denser than water (8.5 lb / gal) and slightly soluble in water. Vapors heavier than air. Toxic by skin absorption and inhalation. Produces toxic oxides of nitrogen during combustion. Used to manufacture other chemicals, especially dyes, photographic chemicals, agricultural chemicals and others. |
|
Air & Water Reactions
|
Darkens on exposure to air and light. Polymerizes slowly to a resinous mass on exposure to air and light. Slightly soluble in water. |
|
Reactivity Profile
|
Aniline is a heat sensitive base. Combines with acids to form salts. Dissolves alkali metals or alkaline earth metals with evolution of hydrogen. Incompatible with albumin, solutions of iron, zinc and aluminum, and acids. Couples readily with phenols and aromatic amines. Easily acylated and alkylated. Corrosive to copper and copper alloys. Can react vigorously with oxidizing materials (including perchloric acid, fuming nitric acid, sodium peroxide and ozone). Reacts violently with BCl3. Mixtures with toluene diisocyanate may ignite. Undergoes explosive reactions with benzenediazonium-2-carboxylate, dibenzoyl peroxide, fluorine nitrate, nitrosyl perchlorate, peroxodisulfuric acid and tetranitromethane. Violent reactions may occur with peroxyformic acid, diisopropyl peroxydicarbonate, fluorine, trichloronitromethane (293° F), acetic anhydride, chlorosulfonic acid, hexachloromelamine, (HNO3 + N2O4 + H2SO4), (nitrobenzene + glycerin), oleum, (HCHO + HClO4), perchromates, K2O2, beta-propiolactone, AgClO4, Na2O2, H2SO4, trichloromelamine, acids, FO3Cl, diisopropyl peroxy-dicarbonate, n-haloimides and trichloronitromethane. Ignites on contact with sodium peroxide + water. Forms heat or shock sensitive explosive mixtures with anilinium chloride (detonates at 464° F/7.6 bar), nitromethane, hydrogen peroxide, 1-chloro-2,3-epoxypropane and peroxomonosulfuric acid. Reacts with perchloryl fluoride form explosive products. |
|
Hazard
|
An allergen. Toxic if absorbed through the
skin. Combustible. Skin irritant. Questionable car-
cinogen. |
|
Health Hazard
|
Aniline is a moderate skin irritant, a moderate to severe eye irritant, and a skin sensitizer
in animals. Aniline is moderately toxic via inhalation and ingestion. Symptoms of
exposure (which may be delayed up to 4 hours) include headache, weakness, dizziness,
nausea, difficulty breathing, and unconsciousness. Exposure to aniline results in the
formation of methemoglobin and can thus interfere with the ability of the blood to
transport oxygen. Effects from exposure at levels near the lethal dose include
hypoactivity, tremors, convulsions, liver and kidney effects, and cyanosis.
Aniline has not been found to be a carcinogen or reproductive toxin in humans. Some
tests in rats demonstrate carcinogenic activity. However, other tests in which mice,
guinea pigs, and rabbits were treated by various routes of administration gave negative
results. Aniline produced developmental toxicity only at maternally toxic dose levels but
did not have a selective toxicity for the fetus. It produces genetic damage in animals and
in mammalian cell cultures but not in bacterial cell cultures. |
|
Fire Hazard
|
Combustion can produce toxic fumes including nitrogen oxides and carbon monoxide. Aniline vapor forms explosive mixtures with air. Aniline is incompatible with strong oxidizers and strong acids and a number of other materials. Avoid heating. Hazardous polymerization may occur. Polymerizes to a resinous mass. |
|
Flammability and Explosibility
|
Aniline is a combustible liquid (NFPA rating = 2). Smoke from a fire involving
aniline may contain toxic nitrogen oxides and aniline vapor. Toxic aniline vapors are
given off at high temperatures and form explosive mixtures in air. Carbon dioxide or
dry chemical extinguishers should be used to fight aniline fires. |
|
Chemical Reactivity
|
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water and rinse with dilute acetic acid; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
Safety Profile
|
Suspected carcinogen
with experimental neoplastigenic data. A
human poison by an unspecified route.
Poison experimentally by most routes
incluhng inhalation and ingestion.
Experimental reproductive effects. A skin
and severe eye irritant, and a rmld sensitizer.
In the body, aniline causes formation of
methemoglobin, resulting in prolonged
anoxemia and depression of the central
nervous system; less acute exposure causes
hemolysis of the red blood cells, followed by
stimulation of the bone marrow. The liver
may be affected with resulting jaundice.
Long-term exposure to a d n e dye
manufacture has been associated with
malignant bladder growths. A common air
contaminant, A combustible liquid when
exposed to heat or flame. To fight fire, use
alcohol foam, CO2, dry chemical. It can
react vigorously with oxidizing materials.
When heated to decomposition it emits
highly toxic fumes of NOx. Spontaneously
explosive reactions occur with
benzenediazonium-2-carboxylate, dibenzoyl
peroxide, fluorine nitrate, nitrosyl
perchlorate, red fuming nitric acid,
peroxodisulfuric acid, and
tetranitromethane. Violent reactions with
boron trichloride, peroxyformic acid,
dhsopropyl peroxydicarbonate, fluorine,
trichloronitromethane (145℃), acetic
anhydride, chlorosulfonic acid,
hexachloromelamine, (HNO3 + N2O4 +
H2SO4), (nitrobenzene + glycerin), oleum,
(HCHO + HClO4), perchromates, K2O2, ppropiolactone,
AgClO4, Na2On, H2SO4,
trichloromelamine, acids, peroxydisulfuric
acid, F03Cl, diisopropyl peroxy-dicarbonate,
n-haloimides, and trichloronitromethane.
Ignites on contact with sodium peroxide +
water. Forms heator shock-sensitive
explosive mixtures with anhnium chloride (detonates at 240°C/7.6 bar), nitromethane,
hydrogen peroxide, 1 -chloro-2,3-
epoxypropane, and peroxomonosulfuric
acid. Reactions with perchloryl fluoride,
perchloric acid, and ozone form explosive
products. |
|
Carcinogenicity
|
The IARC has classified aniline as a Group 3 carcinogen,
that is, not classifiable as to its carcinogenicity. However,
NIOSH has determined that there is sufficient evidence
to recommend that OSHA require labeling this substance a
potential occupational carcinogen. This position followed an
evaluation of a high-dose feeding study of aniline hydrochloride in F344 rats and B6C3F1 mice (3000 or
6000 ppm and 6000 or 12,000 ppm, respectively). The test
was negative in both sexes of mice; however, hemangiosarcomas
of the spleen and combined incidence of fibrosarcomas
and sarcomas of the spleen were statistically significant
in the male rats; the number of female rats having fibrosarcomas
of the spleen was also significant. |
|
Source
|
Detected in distilled water-soluble fractions of regular gasoline (87 octane) and Gasohol
at concentrations of 0.55 and 0.20 mg/L, respectively (Potter, 1996). Aniline was also detected in
82% of 65 gasoline (regular and premium) samples (62 from Switzerland, 3 from Boston, MA). At
25 °C, concentrations ranged from 70 to 16,000 μg/L in gasoline and 20 to 3,800 μg/L in watersoluble
fractions. Average concentrations were 5.8 mg/L in gasoline and 1.4 mg/L in watersoluble
fractions (Schmidt et al., 2002).
Based on laboratory analysis of 7 coal tar samples, aniline concentrations ranged from ND to 13
ppm (EPRI, 1990).
Aniline in the environment may originate from the anaerobic biodegradation of nitrobenzene
(Razo-Flores et al., 1999). |
中文
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego